Microscopic and thermodynamic analysis of PEG–β-lactoglobulin interaction
Abstract
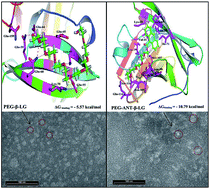
We report the binding of milk β-lactoglobulin (β-LG) with PEG-3000, PEG-6000 and methoxypoly(ethylene glycol) anthracene (mPEG-anthracene) in aqueous solution at pH 7.4, using multiple spectroscopic methods, thermodynamic analysis, transmission electron microscopy (TEM) and molecular modeling. Thermodynamic and spectroscopic analysis showed that polymers bind β-LG via van der Waals interactions, hydrogen bonding and hydrophobic interactions, with overall binding constants KPEG-3000–β-LG = 9.2 (±0.9) × 103 M−1, KPEG-6000–β-LG = 9.7 (±0.7) × 103 M−1 and KmPEG-anthracene–β-LG = 5.5 (±0.5) × 104 M−1. The binding affinity was mPEG-anthracene > PEG-6000 > PEG-3000. Transmission electron microscopy analysis showed significant changes in protein morphology as polymer–protein complexation occurred, with a major increase in the diameter of the protein aggregate. Modeling showed several hydrogen bonding systems between PEG and the different amino acid stabilized polymer–β-LG complexes. The free binding energy indicated that the interaction process is spontaneous at room temperature. Furthermore, mPEG-anthracene is a stronger protein binder than PEG-3000 and PEG-6000, due to its major hydrophobic characteristics.
- This article is part of the themed collection: Molecular modelling

 Please wait while we load your content...
Please wait while we load your content...