Sustainable one-pot aqueous route to hierarchical carbon–MoO2 electrodes for Li-ion batteries†
Abstract
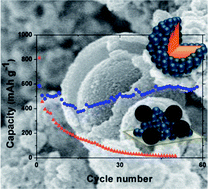
A route towards carbon–MoO2 core–shell spheres has been developed, through hydrothermal decomposition of ascorbic acid combined with precipitation of MoO2 nanoparticles. In this one-pot and green process, carbon spheres originating from ascorbic acid act as seeds for the in situ deposition of a corona made of 30 nm molybdenum dioxide particles. The as-obtained hierarchical nanostructured carbon–MoO2 core–shell spheres exhibit an ideal combination of electrical conductivity and lithium reactivity for Li-ion battery electrodes. This nanocomposite offers the opportunity to master the collector-active material and active material–electrolyte interfaces. Direct transfer “from the beaker to the battery” without any additives nor thermal treatment yields storage capacity values of ca. 600 mA h·g−1 at C/5 rate with excellent stability that challenges state-of-the-art molybdenum oxide-based batteries.

 Please wait while we load your content...
Please wait while we load your content...