Incorporating Ce3+ into a high efficiency phosphor Ca2PO4Cl:Eu2+ and its luminescent properties†
Abstract
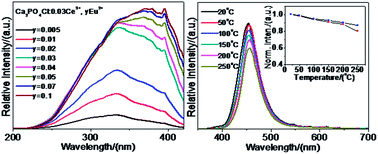
A series of Ce3+, Eu2+ and Ce3+/Eu2+ doped Ca2PO4Cl phosphors are synthesized by a high temperature solid-state method. Not only is the emission intensity of Ca2PO4Cl:Eu2+ obviously enhanced by codoping Ce3+, but also the spectral profile of the excitation band is almost not influenced. The energy transfer from Ce3+ to Eu2+ in Ca2PO4Cl has been validated and proved to be a resonant type via a dipole–dipole interaction. Under the 400 nm radiation excitation, the luminescent intensity of Ca2PO4Cl:Ce3+, Eu2+ is found to be about 200% that of Ca2PO4Cl:Eu2+, and 300% that of BaMgAl10O17:Eu2+. The thermal quenching properties reveal that Ca2PO4Cl:Ce3+, Eu2+ has excellent characteristics. Therefore, Ca2PO4Cl:Ce3+, Eu2+ may have potential application as a blue-emitting phosphor for white LEDs.

 Please wait while we load your content...
Please wait while we load your content...