BiFeO3/YSZ bilayer electrolyte for low temperature solid oxide fuel cell†
Abstract
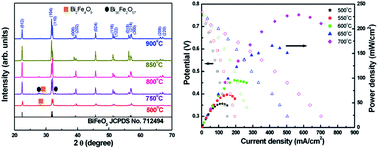
We have demonstrated BiFeO3 (BFO) as a potential bilayer electrolyte for 650 °C low temperature solid oxide fuel cell application. The stoichiometric perovskite BFO is synthesized by wet chemistry, calcined at 500 °C and sintered at 850 °C. The crystalline structure is confirmed by X-ray diffraction spectroscopy, the atomic ratios (Bi : Fe) of 1.02 and 1.00 are determined by X-ray energy dispersive spectroscopy and inductively coupled plasma-mass spectroscopy, respectively. The X-ray photoelectron spectroscopy analysis indicates the presence of oxygen vacancies which can partially reduce Fe3+ and result in relatively high dielectric constant (6252 at 100 kHz) and ionic conductivity (>10−2 S cm−1 at 650 °C). The BFO is coated with an yttria-stabilized zirconia (YSZ) protective layer to avoid hydrogen reduction of BFO. This bilayer electrolyte exhibits a 1.6 times increase in maximum power density as compared with pure YSZ when a Ni–YSZ anode and lanthanum strontium cobalt ferrite (LSCF) cathode are used in the fuel cell at 650 °C.

 Please wait while we load your content...
Please wait while we load your content...