Facile synthesis of fructone from ethyl acetoacetate and ethylene glycol catalyzed by SO3H-functionalized Brønsted acidic ionic liquids
Abstract
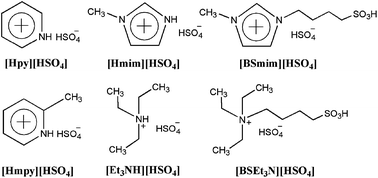
SO3H-functionalized Brønsted acidic ionic liquids (BAILs) were synthesized and utilized as highly efficient catalysts for the production of fructone via the acetalization reaction of ethyl acetoacetate with ethylene glycol. In comparison with conventional H2SO4 and cation exchange resins, the BAILs N-(4-sulfonic acid) butyl triethylammonium hydrogensulfate ([BSEt3N][HSO4]) of strong acidities exhibited excellent catalytic activities. The effects of various parameters such as different BAILs, reaction temperature, catalyst dosage, and molar ratio of the reactants on the conversion of ethyl acetoacetate were investigated in detail. The experimental results indicated that the catalytic performance of BAILs were closely related to their Hammett acidities. Moreover, it was found that [BSEt3N][HSO4] could be also recovered easily and used repetitively six times without obvious decline in activity and quantity, showing great potential application in industry.

 Please wait while we load your content...
Please wait while we load your content...