InCl3-catalyzed Prins bicyclization for the synthesis of spirotetrahydropyran derivatives†
Abstract
A novel InCl3 catalyzed Prins bicyclization strategy has been developed for the synthesis of tetrahydro-3H-spiro[benzo[b][1,4]dioxine-2,4′-pyran] and hexahydrospiro[benzo[b][1,4]oxazine-2,4′-pyran] derivatives. The spirocyclization of 2-(4-hydroxy-2-methylenebutoxy)phenol with aldehyde proceeds at room temperature with high diastereoselectivity, whereas the similar cyclization with N-(4-hydroxy-2-methylenebutyl)-N-(2-hydroxyethyl)-4-methylbenzenesulfonamide requires −20 °C to afford the product with good diastereoselectivity.
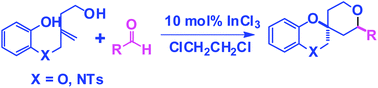

 Please wait while we load your content...
Please wait while we load your content...