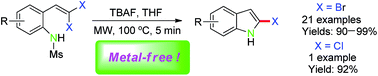
Microwave irradiated synthesis of 2-bromo(chloro)indoles via intramolecular cyclization of 2-(gem-dibromo(chloro)vinyl)anilines in the presence of TBAF under metal-free conditions†
Abstract
2-Bromo(chloro)indoles were readily and efficiently prepared through TBAF-promoted intramolecular cyclization of 2-(gem-dibromo(chloro)vinyl)anilines in excellent yields under metal-free and microwave irradiation conditions.

 Please wait while we load your content...
Please wait while we load your content...