Method for the synthesis of N-alkyl-O-alkyl carbamates†
Abstract
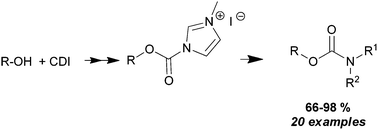
Primary and secondary carbamates were prepared from primary, secondary and neo-pentyl alcohols, and carbonyl-di-imidazole (CDI), using a new three step method. Non-acidic alcohols reacted with CDI affording carbamoyl-imidazoles with excellent yields (92–97%). The carbamoyl-imidazoles were then converted into the more reactive imidazolium salts, which upon reaction with primary and secondary amines afforded the corresponding carbamates in high isolated yields (66–99%).

 Please wait while we load your content...
Please wait while we load your content...