Lewis acid promoted C–C and copper-catalyzed C–O bond formation: synthesis of neoflavans†
Abstract
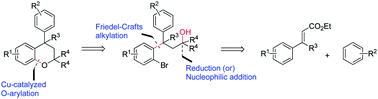
An intramolecular [Cu]-catalyzed C–O bond formation for the synthesis of neoflavans is presented. Lewis acid promoted Friedel–Crafts Michael addition of electron rich aromatic systems onto the double bond of the cinnamate ester was employed to furnish a β-diaryl ester. Electrophilic aromatic bromination of the β-diaryl ester and reduction/Grignard addition furnished the required precursor alcohols. The method is applicable to the synthesis of neoflavans containing tertiary as well as quaternary carbon centers. Significantly, the neoflavan substructures are present in biologically active compounds.

 Please wait while we load your content...
Please wait while we load your content...