Interaction of acetone with the Ge(001) surface
Abstract
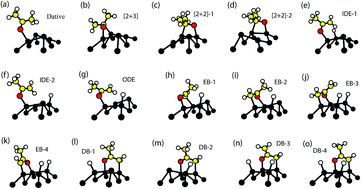
The adsorption of acetone on the Ge(001) surface has been investigated using density functional theory (DFT). We have considered a number of different possible adsorbate configurations corresponding to the dative bonded, α-hydrogen cleavage, [2 + 2] cycloaddition, end-bridging and dimer-bridging structures. Analysis of the associated energetics has resulted in a comprehensive description of the overall reaction processes of acetone on the Ge(001) surface. The preferred structure at low temperature is found to be the dative structure, whilst at higher temperature the predominant structure is predicted to be a dimer-bridge dissociated configuration. Both of these predictions are consistent with the available experimental data. Comparison with earlier work of acetone on Si(001) reveals significantly weaker bonding in the case of Ge(001), and highlights fundamental differences between the chemical properties of these two surfaces.

 Please wait while we load your content...
Please wait while we load your content...