Highly efficient and reversible CO2 capture through 1,1,3,3-tetramethylguanidinium imidazole ionic liquid†
Abstract
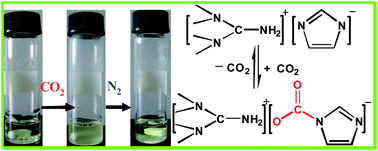
1,1,3,3-Tetramethylguanidinium imidazole ([TMG][IM]) ionic liquid was synthesized and its absorption and desorption of CO2 were investigated, as well as the effect of reaction temperature, N2, and moisture on capture of CO2 and the absorption mechanism. The results show that the maximum molar ratio of CO2 to [TMG][IM] is to achieve 1.01 at 30 °C under atmosphere pressure with a high absorption rate. The capture capability is not obviously affected by the moisture of CO2, but decreases with temperature increasing from 30 to 50 °C. The absorption mechanism might be that CO2 reacts with the imidazole anion of [TMG][IM] and then a new carbamate group is formed, which is confirmed by IR and NMR spectra. The enthalpy of CO2 absorption for [TMG][IM] is −30.3 kJ mol−1. The captured CO2 can be readily released at 65 °C by bubbling N2 and recycled with little loss of its capture capability. Considering the efficient and reversible process with [TMG][IM], this method has great potential for the capture of CO2.

 Please wait while we load your content...
Please wait while we load your content...