Noble gas induced surprisingly higher stability of π hydrogen bonded complex: comparative study of hydrogen bonded complexes of HKrCCH and HCCH with H2O, NH3, CH3OH and CH3NH2
Abstract
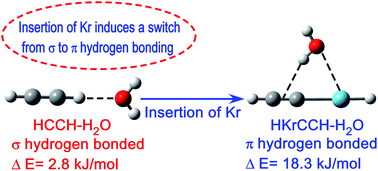
Structure, energetics and spectroscopic properties of hydrogen bonded complexes of HKrCCH with H2O, NH3, CH3OH and CH3NH2 have been explored as well as compared with the hydrogen bonded complexes of its precursor HCCH. The most stable structure for HKrCCH is the π (O/N–H⋯π) hydrogen bonded complex, whereas the σ (C–H⋯O/N) hydrogen bonded complex is most stable for HCCH. Surprisingly, the stabilization energy of the π hydrogen bonded complex of HKrCCH is about two to three fold higher compared to the σ hydrogen bonded complex of HCCH, which is in contrast to the general perception that the strength of π hydrogen bond is less compared to σ hydrogen bond. From the atom in molecule and charge analyses, it has been found that π hydrogen bonded complexes of HKrCCH have a cyclic structure in which O/N–H⋯π hydrogen bonding takes place along with Kr⋯O/N interaction. Higher stabilization energy of the π hydrogen bonded complex of HKrCCH is due to a cooperative phenomena in which Kr⋯O/N interaction contributes to the formation of a strong O/N–H⋯π hydrogen bonding.

 Please wait while we load your content...
Please wait while we load your content...