A concise synthetic approach toward tamiflu (oseltamivir phosphate): cis-aziridine as the key synthon and RCM†
Abstract
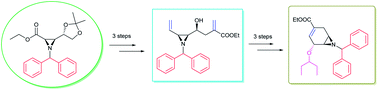
The key synthon cis-aziridine has been efficiently utilised for the synthesis of oseltamivir phosphate, using Wittig olefination, Barbier addition, Mitsunobu reaction and ring closing metathesis (RCM) as key essentials.

 Please wait while we load your content...
Please wait while we load your content...