Mn2+/graphene oxide nanocomposite efficiently catalyzes the epoxidation of alkenes with H2O2
Abstract
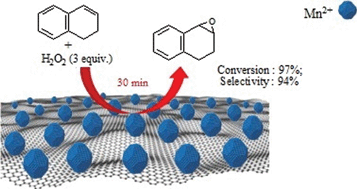
A novel Mn2+/graphene oxide (Mn2+/GO) nanocomposite has been developed by coordination of the Mn2+ ions of Mn(OAc)2·4H2O with the oxygenated functional groups of the GO sheets. Characterization results strongly suggested that Mn2+ ions were dispersed homogeneously on the surface or the space between GO sheets through dative bonds. The unique layered structure of the GO carrier with flexible interlayer spaces favored reagents' diffusion towards catalytic active sites, and therefore resulted in high efficiency and good universality of the heterogeneous Mn2+/GO nanocomposite in the epoxidation of alkenes with H2O2. Excellent turnover frequency (TOF) values and selectivities for the epoxides were obtained with a wide range of either bulky or less bulky alkenes (styrene, α-methylstyrene, indene and 1,2-dihydronaphthalene). Furthermore, the Mn2+/GO nanocomposite could be easily recovered and recycled for several times without significant loss of the activity and selectivity.

 Please wait while we load your content...
Please wait while we load your content...