Characterization and applications of cyclic β-(1,2)-glucan produced from R. meliloti†
Abstract
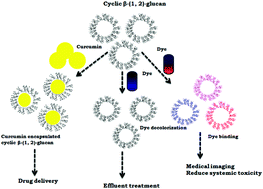
Cyclic β-(1,2)-glucan, with a degree of polymerization ranging from 17–28, without any substitution and a molar mass of 3101.5 Da, was produced from Rhizobium Meliloti MTCC-3402 in glutamic acid and a mannitol medium. The size of this glucan was less than those reported from oats and yeast. The main fraction was with 19 glucose residues (cavity size of 0.92 nm) and a melting temperature of 134.1 °C. Glucan encapsulates drugs (85–99%) such as curcumin, dexamethasone, reserpine, 6-methylcoumarin, 4-hydroxycoumarin and 4 methyl umbelliferone very efficiently. The encapsulation efficiency was better for hydrophobic than hydrophilic drugs (correlation coefficient of 0.92). Glucan was not cytotoxic towards L6 myoblast and 3T3 fibroblast cells and could be produced on the nanometer scale (average particle size 50–200 nm). Glucan exhibited dose dependent radical scavenging antioxidant activity. It was able to bind to dyes including methyl violet, trypan blue and bromocresol green indicating that the latter could be used in vivo at very low concentrations. Glucan decolorized coomassie brilliant blue R, bromophenol blue and bromocresol purple opening up applications in effluent treatment industries.

 Please wait while we load your content...
Please wait while we load your content...