On the kinetics and reaction mechanisms of boronic acid in interaction with diols for non-enzymatic glucose monitoring applications: a hybrid DFT study
Abstract
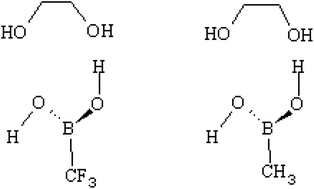
Boronic acids are well-known for their ability to complex with saccharides and to form cyclic boronic esters or cyclic boronate ions. Increasing the reactivity of boronic acids would lead towards the design of a highly responsive sensor for non-enzymatic glucose monitoring applications. Many studies have been carried out to investigate the reactivity of boronic acids towards diols in different environmental effects. The symmetry around boron in their reactive species, however, is still an open question. In this study, we used computational quantum chemistry calculations to propose a model in which boronic acid is highly reactive towards diols. B3LYP/6-31+G(d,p) model chemistry was used in water to calculate the transition states of several proposed reaction mechanisms and the rates of the reactions were calculated using the transition state theory. The reaction takes place in two steps with the first step known as the rate determining step. The model we proposed in this study includes two different electronegative R-groups attached to boronic acid (i.e. CF3 and CH3) which highly influence the interaction of boron with diols. The kinetic results in our study show that boronate ion with a low electronegative R-group is a better sensor in alkaline medium and boronic acid with a high electronegative R-group is a better sensor in acidic medium. Using the latter in alkaline medium results in a very poor sensor since it is highly reactive and promptly forms a tetrahedral boronate ion. The high electronegative R-group on boronate ion decreases its basic characteristics and results in a low reactive sensor. The same scenario goes for the former i.e. boronate ion attached to a low electronegative R-group in acidic medium. As a result, we can conclude that boronic acid's reactivity is not mainly due to the symmetry around boron, it is due to the characteristics of boron itself which can be affected by both the R-group and the medium.

 Please wait while we load your content...
Please wait while we load your content...