Degradation of anion exchange membranes used for hydrogen production by ultrapure water electrolysis†
Abstract
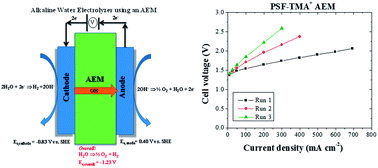
Solid-state alkaline water electrolysis using a pure water feed offers several distinct advantages over liquid alkaline electrolyte water electrolysis and proton exchange membrane water electrolysis. These advantages include a larger array of electrocatalyst available for oxygen evolution, no electrolyte management, and the ability to apply differential pressure. To date, there have been only a handful of reports on solid-state alkaline water electrolyzers using anion exchange membranes (AEMs), and there have been no reports that investigate loss in system performance over time. In this work, a solid-state alkaline water electrolyzer was successfully demonstrated with several types of polysulfone-based AEMs using a relatively expensive but highly active lead ruthenate pyrochlore electrocatalyst for the oxygen evolution reaction. The electrolysis of ultrapure water at 50 °C resulted in a current density of 400 mA cm−2 at 1.80 V. We demonstrated that the short-term degradation of water electrolyzer performance over time was largely a consequence of carbon dioxide intrusion into the system and could be easily remedied, while long-term deterioration was a consequence of irreversible AEM polymer degradation.

 Please wait while we load your content...
Please wait while we load your content...