Towards a sustainable synthesis of aniline-derived amides using an indirect chemoenzymatic process: challenges and successes†
Abstract
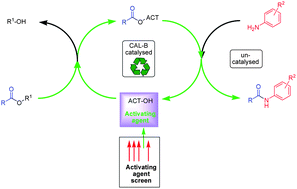
A general, catalytic and sustainable synthesis of amides has been targeted which utilises a chemoenzymatic step to generate an activated amide coupling partner in situ. A screen of a series of known activating agents was performed and the successful agents taken forward to determine their utility in such a one-pot enzyme-catalysed process. In these studies, a new chemoselective reagent ((isopropylideneamino) 2-phenylacetate) has been identified, which demonstrates excellent selectivity for the acylation of primary anilines over secondary anilines, unlike the more conventional acid chloride equivalent, which is unselective. The development of a chemoenzymatic synthesis of 2-hydroxyethyl benzoates (ethylene glycol mono-benzoate esters) has also been achieved.

 Please wait while we load your content...
Please wait while we load your content...