Evaluation of decomposition products of EMImCl·1.5AlCl3 during aluminium electrodeposition with different analytical methods
Abstract
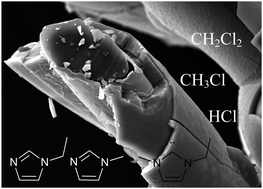
Ionic liquids are of great importance for electrodeposition of metals, which can't be deposited from aqueous electrolytes due to their negative standard potentials. In this paper non-woven polymers were coated with aluminium by electrodeposition from 1-ethyl-3-methyl-imidazolium chloride and subsequently established as 3D current collectors for lithium-ion batteries. We investigated the long-term stability of the ionic liquid (IL) for electrodeposition of aluminium under process-oriented conditions. The degradation products were analysed by headspace gas chromatography-mass spectrometry, pyrolysis-gas chromatography-mass spectrometry (Py-GC/MS) and 1H/13C nuclear magnetic resonance spectroscopy (NMR). The main decomposition products derived from thermal degradation, especially from cleavage of an alkyl chain and were identified as chloromethane, dichloromethane, methylimidazole, ethylimidazole and deprotonated 1-ethyl-3-methylimidazole.

 Please wait while we load your content...
Please wait while we load your content...