Enhanced spectrofluorimetric determination of hypochlorite based on the catalytic oxidation of thiamine to thiochrome in the presence of trace ferrocyanide†
Abstract
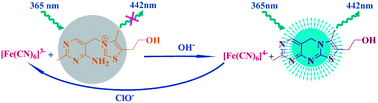
An enhanced spectrofluorimetric method for highly selective and sensitive determination of nmol L−1 of hypochlorite has been performed. In alkaline medium, ferrocyanide was specifically oxidized by hypochlorite to form ferricyanide, which further reacted with non-fluorescent thiamine, resulting in the formation of blue fluorescent thiochrome. Notably, the fluorescence intensities were positively correlated with the concentration of hypochlorite over the range of 0.02–12.5 μmol L−1. The detection limit was 6.1 nmol L−1, which was lower than for most of the recently published methods. The experimental conditions were optimized and effects of coexisting substances were evaluated and the results showed excellent priority since a certain amount of other anions, including ClO3−, BrO3−, IO3−, Cr2O72− and other acid radicals, would not interfere with the measurement.

 Please wait while we load your content...
Please wait while we load your content...