An amino acid-based swift synthesis of zinc oxide nanostructures†
Abstract
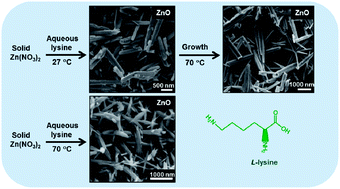
Zinc oxide (ZnO) nanostructures (rods, ellipsoids and flowers) were synthesized using a zinc precursor and L-lysine in aqueous medium at room temperature and/or at elevated temperature. The average thicknesses of different zinc oxide nanorods samples synthesized by this eco-friendly route are in the range of 109 to 291 nm. The synthesized ZnO samples were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The effects of concentration, concentration ratio of reactants, heat, zinc precursor or precipitating agent on the resulting ZnO have been studied. XRD analysis of nanorods proved the formation of hexagonal ZnO. Results revealed that lysine is responsible for the selective formation of 1-D morphologies even at room temperature. Acetate precursor was found to yield thinner nanorods than nitrate precursor. Based on the results, a plausible mechanism of formation of nanostructures has been proposed.

 Please wait while we load your content...
Please wait while we load your content...