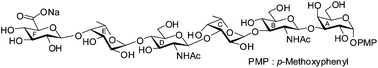
Convergent synthesis of a hexasaccharide corresponding to the cell wall O-antigen of Escherichia coli O41†
Abstract
A convergent synthetic strategy has been developed for the synthesis of a hexasaccharide corresponding to the O-antigen of E. coli O41 using stereoselective [3 + 3] block glycosylation strategy. The target compound was synthesized assembling a series of suitably protected monosaccharide intermediates. Thioglycoside derivatives have been used as glycosyl donors in most of the glycosylation reactions. All intermediate steps are high yielding and the glycosylation steps are highly stereoselective. A number of recently developed methodologies have been used in the synthesis.

 Please wait while we load your content...
Please wait while we load your content...