Diastereoselective self-assembly of heterochiral Zn(ii) complexes of racemic Schiff bases in a chiral self-discriminating process: effect of non-covalent interactions on solid state structural self-assembly†
Abstract
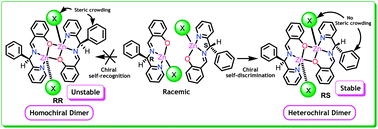
Diastereoselective self-assembly of Zn(II) heterochiral complexes of racemic Schiff bases L1H and L2H (where L1H = 2-((phenyl(2-pyridyl)methylimino)-methyl)phenol; L2H = 1-((phenyl(2-pyridyl)methylimino)methyl)-2-naphthol) in a chiral self-discriminating process are reported. Complexes 1–6 are synthesized using ligand L1H (1–3), L2H (4–6), Zn(NO3)2·6H2O, and co-ligands such as N3− or NCS− and are conclusively structurally characterized. Determination of molecular structures of 1–5 confirmed the presence of a di-metallic core constructed by monochelated tridentate ligands with an inversion centre located directly between the two zinc ions. In 1–5, within one centrosymmetric dimer one of the ligands possessed R configuration whereas other possessed S configuration resulting a heterochiral dimerization of ligands around the Zn(II) centre in a chiral self-discriminating manner. Complex 6 is mononuclear having R configuration of ligand L2 and linked through a C–H⋯O interaction with the nearby mononuclear Zn(II) centre having S configuration of ligand L2 and consequently a heterochiral dimer. The plausible mechanism for the chiral self-discrimination is revealed which makes it clear that trans oriented anions direct the phenyl group appended to the asymmetric carbon atoms to be trans due to steric congestion and consequently the heterochiral dimerization configuration. The effects of different non-covalent interactions to self-assemble the heterochiral dimers to 1D chain having the sequence ⋯RS⋯RS⋯RS⋯ or ⋯RS⋯SR⋯RS⋯ or ⋯R⋯S⋯R⋯ and to transfer the stereochemical information of the heterochiral dimers throughout the chain are studied. Also the effects of the anions on the isotactic and syndiotactic arrangements of coordination complex are studied. The results demonstrate that ligand modification, anions and non-covalent interactions play a domino role on controlling the solid state structural rearrangements and photoluminescence properties of the synthesized complexes.

 Please wait while we load your content...
Please wait while we load your content...