Tailoring of mechanical properties of derivatized natural polyamino acids through esterification and tensile deformation†
Abstract
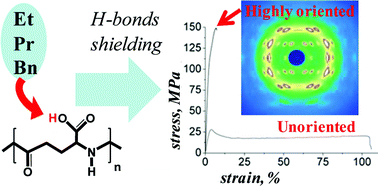
Tensile deformation was applied to the naturally produced poly-γ-glutamic acid, which can be enzymatically degraded and is, therefore, of interest for biomedical use. However, natural polyamino acids have a similar chemical structure to synthetic polyamides (“nylons”), which are known to feature strong inter-molecular hydrogen bonding that prevents large-scale molecular motion in their solid state. Through esterification, this hydrogen bonding was partially shielded, allowing orientation of the polyamino acid macromolecules through tensile deformation. An increase in Young's modulus and tensile strength was achieved of solution-cast films of the chemically modified poly-γ-glutamic acids, consistent with enhanced uniaxial polymer chain orientation. The latter was confirmed by both wide-angle X-ray scattering and polarized Raman spectroscopy. The films thus produced were found to be non-cytotoxic. These mechanically tailorable, biocompatible polymers may be excellent candidates for use in musculoskeletal tissue engineering applications that have different loading requirements within the body.

 Please wait while we load your content...
Please wait while we load your content...