Hydrogen bonding patterns in different acrylamide–water clusters: microsolvation probed by micro Raman spectroscopy and DFT calculations
Abstract
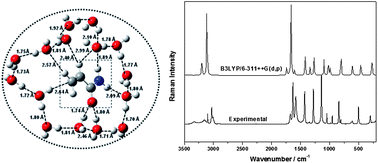
We report in this study on the hydrogen bonding patterns between acrylamide (Acr) and water (W) as a H-donor. Hydrogen bonds between Acr and water molecules and among different water molecules significantly influence the spectral features. Raman spectra of neat Acr and its mixtures with water were recorded in the region, 1800–400 cm−1. A careful analysis of the spectra reveals that upon dilution, the additional peaks are observed at 831 and 1124 cm−1, ∼10 and ∼18 cm−1 away from the main bands at ∼842 and ∼1142 cm−1, respectively, which were attributed to the hydrogen bonding of Acr with water. The new band at ∼1083 cm−1 clearly reveals a nice example of the increment on the degree of hydrogen bonding in terms of multiple hydrogen bonded molecules. The temperature dependent Raman spectra of Acr at nine different temperatures were also recorded, and a significant change in spectral features observed at ∼373 K is attributed to crystal → liquid transition. A new peak at 1620 cm−1 appears to be due to a change in the symmetry of self associated Acr (dimer and trimer) molecules at 373 K. DFT calculations were performed using B3LYP/6-311++G(d,p) to obtain the ground state optimized geometries of neat, self associated dimeric and trimeric forms, and hydrogen bonded complexes in gas phase. The DFT computations were also performed on various (Acr + Wn, n = 1–15) clusters in order to explore the microsolvation. A broad configuration search was performed to identify the lowest energy clusters of Acr with varying number of water molecules. The structures of the clusters are analyzed in terms of the hydrogen bonding network established among the water molecules and between Acr and water molecules. Overall the present study gives a clue regarding the stabilization of the Acr molecule in a large cluster of water molecules.

 Please wait while we load your content...
Please wait while we load your content...