Design, synthesis, and evaluation of substituted 6-amide-4-anilinoquinazoline derivatives as c-Src inhibitors
Abstract
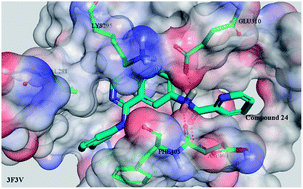
The 4-anilinoquinazoline scaffold has been historically used for designing EGFR/VEGFR/HER2 inhibitors while it has not been reported widely for developing c-Src inhibitors. Thus, a series of novel 4-anilinoquinazoline derivatives grafting different amide moieties at the 6-position were designed and synthesized as potential inhibitors for c-Src. In this manuscript, all of the designed compounds were screened via molecular docking using Discovery Studio 3.5 software. As expected, the results of the docking study revealed that most of these targeted compounds possessed lower binding energy than the positive control Saracatinib. Subsequently, all of the screened compounds were synthesized and evaluated for their c-Src in vitro inhibitory activities and in vitro antiproliferation assays against four human cancer cells (A549, MCF-7, HepG-2, HeLa). Among these compounds, 24 exhibited the most potent inhibitory activity against c-Src kinase as well as at the cellular level, of which the IC50 value reached up to 2.9 nM, comparable to the positive compound Saracatinib. Kinase selectivity profile also demonstrated that compound 24 showed good selectivity over several close kinase targets. These results, along with relative 3D-QSAR study, could provide an important basis for further development of compound 24 as a potent tyrosine kinase inhibitor.

 Please wait while we load your content...
Please wait while we load your content...