Copper(ii) triflate-catalyzed reactions for the synthesis of novel and diverse quinoline carboxylates†
Abstract
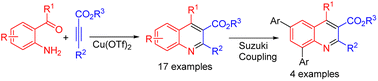
An efficient one-pot synthesis of a variety of quinoline carboxylates was accomplished by Cu(OTf)2-catalyzed reactions of Michael addition/cyclization/aromatization between 2-aminoaryl carbonyls and alkynyl carboxylates. This methodology offers several significant advantages, namely, ease of handling, mild reaction conditions, enhanced reaction rates, and use of an effective and non-toxic catalyst. The synthesized compounds were further transformed into highly functionalized novel quinoline carboxylates bearing aromatic rings on the quinoline skeleton using the Suzuki reaction.

 Please wait while we load your content...
Please wait while we load your content...