Investigation and folding pattern of l-ido and d-gluco peptides by EASY ROESY NMR and X-ray†
Abstract
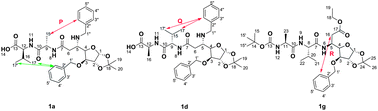
The sugar β-amino acid plays an important role in the formation of well-defined secondary structures of peptides. The combination of change in the functional group, stereocenter on the sugar amino acid and peptide attachment to the glycopeptide revealed important information about their secondary structure. The stereocentre (C5–H) of the sugar β-amino acid in these peptidic diastereomers controls the folding behaviour of these compounds. Conformations of the considered compounds were established by NMR (EASY-ROESY) and compared with the 3D structure obtained from X-ray crystallography.

 Please wait while we load your content...
Please wait while we load your content...