Construction of Cu(ii) coordination polymers based on semi-rigid tetrahedral pyridine ligands†
Abstract
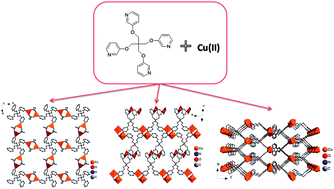
The assembly of a Cu(II) ion and the semi-rigid tetrahedral pyridine ligand, tetrakis(3-pyridyloxymethylene)methane (L1), yields three coordination polymers (1–3) by synergy between different solvent mixtures. Single crystal X-ray diffraction analyses reveal that Cu4(L1)(OAc)8 (1) features a two-dimensional sql network, and comprises dimeric five-coordinated Cu-centered square pyramids as the inorganic building units; Cu2(L1)(C7H5O2)4 (2) is also a layered assembly but with a 3,4-connected 3,4L13 net. In this complex, mononuclear CuO2N2 square planar structures and dinuclear CuO3N2 square pyramids both serve as the inorganic building units. Cu(H2O)2(L1)(OAc)2 (3) is a three-dimensional framework structure based on six-coordinated CuO2N4 square bipyramidal inorganic nodes and tetrahedral semi-rigid pyridine ligands, and dia topology is simplified for 3.

 Please wait while we load your content...
Please wait while we load your content...