Product analysis of photooxidation in isolated quadruplex DNA; 8-oxo-7,8-dihydroguanine and its oxidation product at 3′-G are formed instead of 2,5-diamino-4H-imidazol-4-one†
Abstract
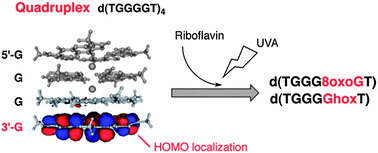
The formation of quadruplex structure changed the site reactivity and the kinds of guanine photooxidation products of d(TGGGGT). In quadruplex DNA, 8-oxo-7,8-dihydroguanine (8oxoG) and dehydroguanidinohydantoin (Ghox) were mainly formed, although 2,5-diamino-4H-imidazol-4-one (Iz) was mainly formed in single-stranded DNA. In addition, 3′-guanine was specifically oxidized in quadruplex DNA compared with single-stranded DNA, which depended on the localization of the HOMO.

 Please wait while we load your content...
Please wait while we load your content...