The influence of hydrophobic/hydrophilic balance of the mesoporous solid acid catalysts in the selective dehydration of fructose into HMF†
Abstract
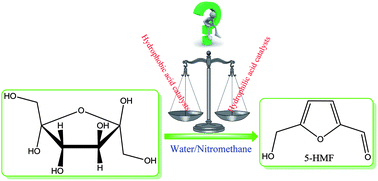
The application of a number of mesoporous sulfonic acids including MCM-41–PrSO3H (1a), MCM-41–Ph–PrSO3H (1b), SBA-15–PrSO3H (2a), SBA-15–Ph–PrSO3H (2b), SBA-15–EtPhSO3H (3a), and SBA-15–Ph–EtPhSO3H (3b) with different surface hydrophobicity in acid-catalyzed dehydration of fructose into 5-hydroxymethylfurfural (HMF) in a water–nitromethane solvent mixture was investigated. Among the studied mesoporous solid acids, the catalysts with lower surface hydrophobicity and relative acidity were found to be more selective and efficient catalytic system for HMF production. Our studies showed that the hydrophilic surface of the employed solid acids might indeed provide a means of faster departure of HMF from the system mesopores as soon as it forms, thus retarding the rehydration of HMF to unwanted by-products and improving the HMF selectivity. HMF selectivity of 75% was reached in 93% fructose conversion in a water–NM system at 140 °C within 30 min in the presence of catalyst 2a, which is comparable or even superior to other systems which employ MIBK–2-butanol or other organic co-solvents.

 Please wait while we load your content...
Please wait while we load your content...