Substituent effects on cyclic electron delocalization in symmetric B- and N-trisubstituted borazine derivatives†
Abstract
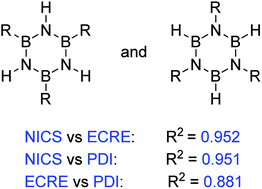
Aromaticity is an important concept in chemistry, useful to rationalize structure, physical properties and chemical behaviour of molecules. Aromaticity of an inorganic relative of benzene, borazine, has been the topic of a number of studies which resulted in its description as weakly aromatic or nonaromatic. However, influence of substituents on its aromatic character is poorly understood. This study shows that an appropriate choice of aromaticity indices can establish a connection between substituent effects and aromaticity of the ring. The changes in cyclic π electron delocalization (aromaticity) were traced by means of the most refined NICS(0)πzz index, the electron delocalization-based index PDI and extra cyclic resonance energy ECRE, computed by using the Natural Bond Orbital (NBO) method. Although various indices often do not correlate well with each other, owing to the multidimensional nature of aromaticity, these three indices agreed quite well. However, the HOMA index failed to give reliable information in this case.

 Please wait while we load your content...
Please wait while we load your content...