An enantioselective approach to (−)-aphanorphine featuring a stereoselective oxidative amidation†
Abstract
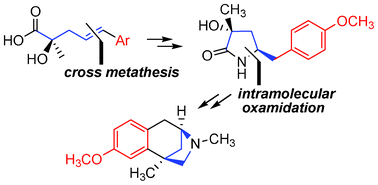
A formal enantioselective synthesis of (−)-aphanorphine (91% ee), that culminates with the preparation of (+)-O-methyl aphanorphine, was achieved. The methodology involves the diastereoselective synthesis of a key 3,5-disubstituted pyrrolidinone intermediate by the intramolecular oxidative amidation of a suitably functionalized α-hydroxy pentenoic acid derivative. A late-stage N-formyl protection, which functions as a latent N-methyl group, is utilized as a simple alternative to a protecting group switch and subsequent N-methylation strategy implemented in all other syntheses of aphanorphine related to the present approach.

 Please wait while we load your content...
Please wait while we load your content...