One-pot sequential reactions for the synthesis of versatile 11C-labeled olefin frameworks†
Abstract
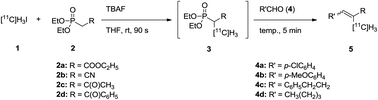
A simple and efficient methodology to construct 11C-labeled olefins (5) was developed. The method involves two different C–C bond-forming reactions: 11C-methylation of a phosphonate (2) using [11C]methyl iodide (1) and subsequent HWE reaction of 11C-methylated phosphonate (3) with aldehydes (4). Tetrabutylammonium fluoride promoted both C–C bond-forming reactions efficiently as a base. This sequential reaction was conducted in one pot. A wide variety of α-[11C]methyl-α,β-unsaturated compounds were prepared by changing the substituents on the phosphonate and the aldehyde.

 Please wait while we load your content...
Please wait while we load your content...