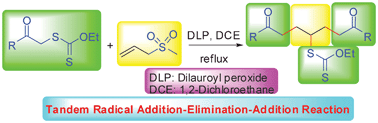
One-pot synthesis of symmetric 1,7-dicarbonyl compounds via a tandem radical addition–elimination–addition reaction†
Abstract
A novel approach to synthesize symmetric 1,7-dicarbonyl compounds via a tandem radical addition–elimination–addition reaction of S-carbonylmethyl xanthates with

 Please wait while we load your content...
Please wait while we load your content...