Additive-free solvothermal synthesis of hierarchical flower-like LiFePO4/C mesocrystal and its electrochemical performance
Abstract
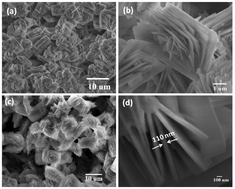
Three dimensional hierarchical flower-like lithium iron phosphate (LiFePO4) mesocrystals were successfully synthesized via a solvothermal approach with the utilization of a mixture of water/ethylene glycol/dimethylacetamide (H2O/EG/DMAC) as co-solvent. No other surfactant or template agent was used and beautiful micro-sized LiFePO4 mesoporous structures with a special rose-like morphology were obtained. The hierarchical LiFePO4 mesocrystals were assembled by well crystallized nano-sized LiFePO4 thin plates with a thickness around 100 nm. The characteristics and electrochemical dynamics as well as performance of the obtained hierarchical flower-like LiFePO4 mesocrystals were carefully investigated. The flower-like hierarchical LiFePO4 mesocrystals showed a high initial lithium intercalation capability of 147 mA h g−1 at a current density of 17 mA g−1 (0.1 C), which should be attributed to the high specific surface area resulting from the mesoporous superstructure and well crystallized LiFePO4 nano-plate composition units. Polyvinylpyrrolidone (PVP) was introduced during the solvothermal synthesis as an in situ carbon coating source. The obtained flower-like C-coated LiFePO4 mesocrystals exhibited even better initial lithium intercalation capability of 161 mA h g−1 at 0.1 C and showed improved lithium storage performance at high rates as well as good cyclic stability.

 Please wait while we load your content...
Please wait while we load your content...