Catalyst-free stereoselective cyclopropanation of electron deficient alkenes with ethyl diazoacetate†
Abstract
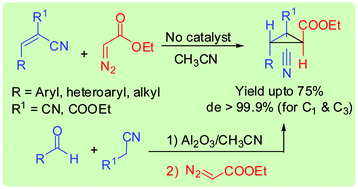
Doubly activated electron deficient alkenes react with ethyl diazoacetate in a Michael Initiated Ring Closure (MIRC) fashion to yield highly diastereoselective cyclopropanes even without any added base or metal catalyst. Following the strategy, a one-pot, two-step, three-component reaction of aldehydes, malononitrile/ethyl cyanoacetate, and ethyl diazoacetate was successfully developed.

 Please wait while we load your content...
Please wait while we load your content...