Organocatalytic enantioselective conjugate addition of nitromethane to alkylidenemalonates: asymmetric synthesis of pyrrolidine-3-carboxylic acid derivatives†
Abstract
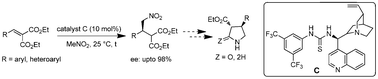
A highly enantioselective nitromethane addition to alkylidene malonates catalyzed by cinchona-

 Please wait while we load your content...
Please wait while we load your content...