Enhanced high rate performance of α-Fe2O3nanotubes with alginate binder as a conversion anode†
Abstract
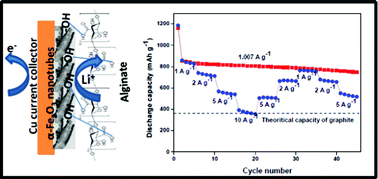
Interactive binders are of current interest to the lithium-ion battery community because they are beneficial for alloy-based anodes. They can accommodate the extra stress generated during the reaction with lithium well and alleviate the pulverization problem associated with the alloying–dealloying process. One of the best examples of an interactive binder is sodium alginate, which has recently being used in silicon-based anodes. The silicon–alginate binder combination has exhibited excellent electrochemical reactivity and stability. Herein, we have utilized the interactive properties of the alginate binder along with the hollow nanostructural features of α-Fe2O3

 Please wait while we load your content...
Please wait while we load your content...