Morphology control of YMn2O5nanocrystals by hydrothermal synthesis and their magnetic properties
Abstract
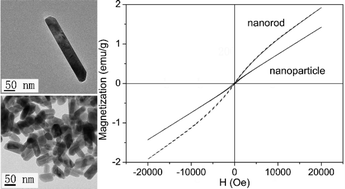
Single-crystalline YMn2O5 was successfully synthesized through a tunable hydrothermal route. The pH value of the growth solution was a crucial factor for the control of the size and morphology of YMn2O5. A low pH value led to the formation of

 Please wait while we load your content...
Please wait while we load your content...