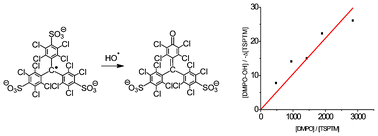
The tripotassium salt of tris(2,3,5,6-tetrachloro-4-hydroxysulfonylphenyl)methyl radical (3 K+ TSPTM3−) reacts in water with hydroxyl radical very fast with a rate constant (k = 2.4 × 1011 M−1 s−1) much higher than that of the reaction of hydroxyl radical with the spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (k = 3.4 × 109 M−1 s−1). The reaction affects the radical character of the molecule and is monitored by electron paramagnetic resonance (EPR). The hydroxyl radical scavenging is established by the characterization of the resulting water-soluble product as the dipotassium salt of 4-[bis(2,3,5,6-tetrachloro-4-hydroxysulfonylphenyl)methylene]-2,3,5,6-tetrachlorocyclohexa-2,5-dien-1-one (2).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...