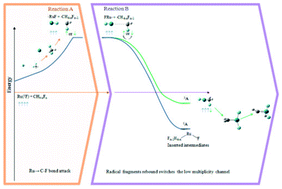
The results obtained from a CASSCF-MRMP2 study of Ru(5F,3F) + CH4−nFn (n = 2–4) reactions are used to propose a two-reaction model that explains the high-oxidation state products, which primarily include carbene and carbyne complexes, detected for these interactions in infrared matrix-isolation determinations, without invoking intersystem crossings between electronic states of different multiplicities. For each of these reactions, it was determined that the channels emerging from the ground and first excited states of the reactants both evolve nearly degenerate radical asymptotes involving the species ·Ru–F and ·CH4−nFn−1 that differ only in the spin of the non-metal fragment. Therefore, under cryogenic-matrix conditions, the caged radicals produced from the ground state of the reactants can recombine and yield species that exhibit quintuplet and triplet multiplicities. The calculated energy profiles for the recombination reactions show that the low-spin channels evolve to the products under matrix conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...