Highly stable acid–base complex membrane for ethanol dehydration by pervaporation separation†
Abstract
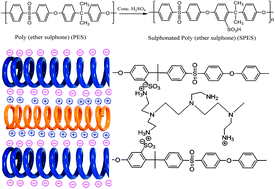
An investigation has been performed into the pervaporation (PV) of the water–ethanol azeotrope using sulphonated poly(ethersulphone) (SPES)–polyethyleneimine (PEI) membranes, the performance of which is compared to the SPES membrane. The SPES–PEI membranes showed high selectivity because of their alternating electrostatic deposition which yields ultrathin, water-selective polyelectrolyte membranes. The thermal and mechanical stabilities of the prepared membranes have been investigated using DSC, TGA, DMA and their structural properties by SEM. The addition of the functional group has been confirmed by solid state 13C NMR and FTIR spectroscopy. The thermal and mechanical data showed that the membranes are mechanically stable up to 250 °C. The glass transition temperature and peak sharpness increases with the quantity of the PEI component, which indicates a rise in the molecular hybridization. FTIR spectra confirm the addition of various functional groups in SPES due to the addition of PEI. The membranes containing 20% (w/w) PEI exhibited the highest water–ethanol selectivity, of the order of 4185 and a flux of 1.2 kg m−2 h−1 at 30 °C.

 Please wait while we load your content...
Please wait while we load your content...