Thermal stability of Li1−ΔM0.5Mn1.5O4 (M = Fe, Co, Ni) cathodes in different states of delithiation Δ
Abstract
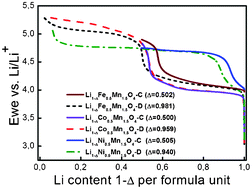
The thermal stability of sol–gel synthesized Li1−ΔM0.5Mn1.5O4 (M = Fe, Co, Ni) electrodes with different degrees of delithiation were analyzed with TG-DSC and in situ synchrotron diffraction under an Ar atmosphere and compared. The onset temperatures for structural degradation are dependent on the amount of lithium 1−Δ in the sample. The Li1−ΔFe0.5Mn1.5O4

 Please wait while we load your content...
Please wait while we load your content...