New clicked full agonists of the estrogen receptor β†
Abstract
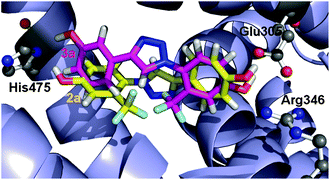
A click chemistry approach was used to synthesize a series of 1,4-diaryl-substituted 1,2,3-triazoles designed to behave as estrogen receptor (ER) ligands. We studied their affinities for both receptors α and β, their agonist activities in a cell-based luciferase reporter assay and their effect on the proliferation of the hormone-dependent MCF-7 cell line. We found two compounds (3a and 3c) that behave as selective full agonists for ERβ at a 20 μM concentration, and one of them (3c) showed no proliferative effect on MCF-7 cells.

 Please wait while we load your content...
Please wait while we load your content...