Ni–Fe–Ce(Mn,Fe)O2 cermet anode for rechargeable Fe–Air battery using LaGaO3 oxide ion conductor as electrolyte†
Abstract
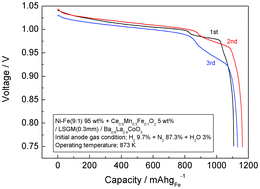
There is a strong demand for the development of a large capacity rechargeable battery in various fields. Recently, we proposed the combination of solid oxide fuel cell technology with Fe–air battery concepts using H2/H2O as a redox mediator and a LaGaO3-based oxide as an electrolyte. Because large internal resistance and large degradation during charge and discharge cycles were observed on the anode, there is a strong demand for improvements in discharge potential and cycle stability. This study investigates the use of a cermet anode consisting of a Ni–Fe alloy combined with an oxide ion conductor. It was observed that by using a cermet anode of Ni–Fe combined with Ce0.6Mn0.3Fe0.1O2 (CMF), the observed capacity of the cell was improved to 1163 mAh g−1 Fe−1 at 10 mA cm−2 and 873 K. This is about 97% of the theoretical capacity by assuming the formation of Fe3O4 (1200 mAh g−1 Fe−1). Cycle stability of the cell was also considerably improved with the use of a Ni–Fe–CMF anode compared to a Ni–Fe anode because of the suppressed aggregation provided by the mixing of Ni with CMF.

 Please wait while we load your content...
Please wait while we load your content...