Facile synthesis and Li-storage performance of SnO nanoparticles and microcrystals
Abstract
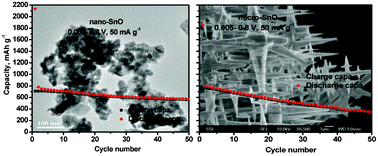
Nano- and micron-sized SnO samples are prepared by simple chemical methods and their lithium cycling behavior is investigated and compared. SnO microcrystals with stacked mesh like morphology are prepared from SnF2 by an aqueous solution synthetic route. SnO

 Please wait while we load your content...
Please wait while we load your content...