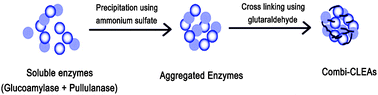
Herein we report the successful preparation of carrier free co-immobilized form of glucoamylase and pullulanase using combi-CLEAs method from commercial multi-enzyme preparation OPTIMAX® 7525 HP. Glucoamylase and pullulanase were precipitated using ammonium sulfate, followed by cross-linking for 8 h with 2% (v/v) glutaraldehyde to produce insoluble catalytically active co-immobilized form of enzymes. The effects of precipitant type and cross-linking were studied and the biocatalyst was characterized. Scanning electron microscopy analysis showed that co-immobilized form of enzymes was of spherical structure. For starch hydrolytic activity, shift in optimum pH from 5 to 7 and temperature from 60 to 70 °C were observed after co-immobilization of enzymes. After starch hydrolysis reaction in batch mode, 100, 80 and 30% conversions were obtained with co-immobilized enzymes, mixture of separate CLEAs and free enzymes, respectively. Co-immobilization also enhanced the thermal stability and storage stability. After eight reuses with 30 min reaction time, co-immobilized glucoamylase and pullulanase retained 90 and 85% of its initial activity, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...