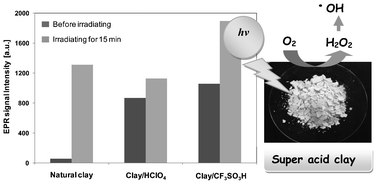
The reactivity of both natural montmorillonite and montmorillonite modified with super acids (CF3SO3H and HClO4) to generate hydrogen peroxide (H2O2) and hydroxyl radicals (˙OH) under both darkness and UV-light irradiation was investigated using EPR spin-trapping spectroscopy. We used the free-radical trapping action of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) to indicate the production of ˙OH from natural and modified clays. The recognition and characterisation of the formed DMPO–OH adduct established that super acid montmorillonites were able to generate hydroxyl radicals at 25 °C in the presence of O2 and H2O. When natural and acid montmorillonite were irradiated with UV light, hydroxyl radical generation was greater than under dark conditions. In addition, the peroxidation of lipids in biological matrices with natural and super acid montmorillonite was studied using a thiobarbituric acid reactive substances (TBARS) assay, which demonstrated that only ˙OH generated from the super acid montmorillonite induced the oxidative degradation of lipids. This study reports, for the first time, the generation of ˙OH and the peroxidation of lipids by a typical super acid-modified Mexican montmorillonite.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...