Evidence of high rate visible light photochemical decolourisation of Rhodamine B with BiFeO3 nanoparticles associated with BiFeO3 photocorrosion†‡
Abstract
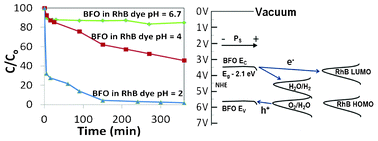
BiFeO3 nanopowders with a size distribution around 20 nm and optical absorption onset at 2.1 eV have been synthesized using self-combustion. These particles were used to photodecolourise Rhodamine B (RhB) dye under a solar simulator with AM1.5 irradiation. XRD analysis after illumination showed no change while XPS analysis on the particles showed significant changes to the chemical state of the Fe cations in BiFeO3 after irradiation. After 10 min of visible (AM 1.5) illumination, at pH 2, RhB showed >95% decolourisation, a rate of decolourisation that rivals other semiconductor systems. We conclude that BiFeO3 nanopowder is a highly active visible light reagent that rivals many of the doped titania systems in terms of activity, but it is not photostable due to photocorrosion of the material during illumination.

 Please wait while we load your content...
Please wait while we load your content...